Adhesion & Cohesion
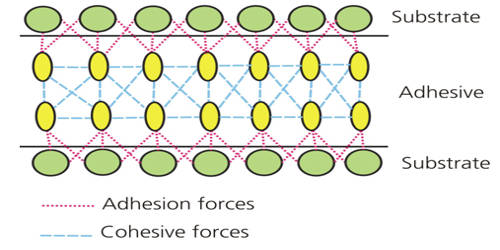
The Principles of Adhesion & Cohesion
Cohesion is defined as the internal strength of an adhesive as a result of a variety of interactions within the adhesive. Adhesion is the bonding of one material to another, namely an adhesive to a substrate, due to a variety of possible interactions. The figure below illustrates adhesion and cohesion forces present within an adhesive and between an adhesive and substrate.
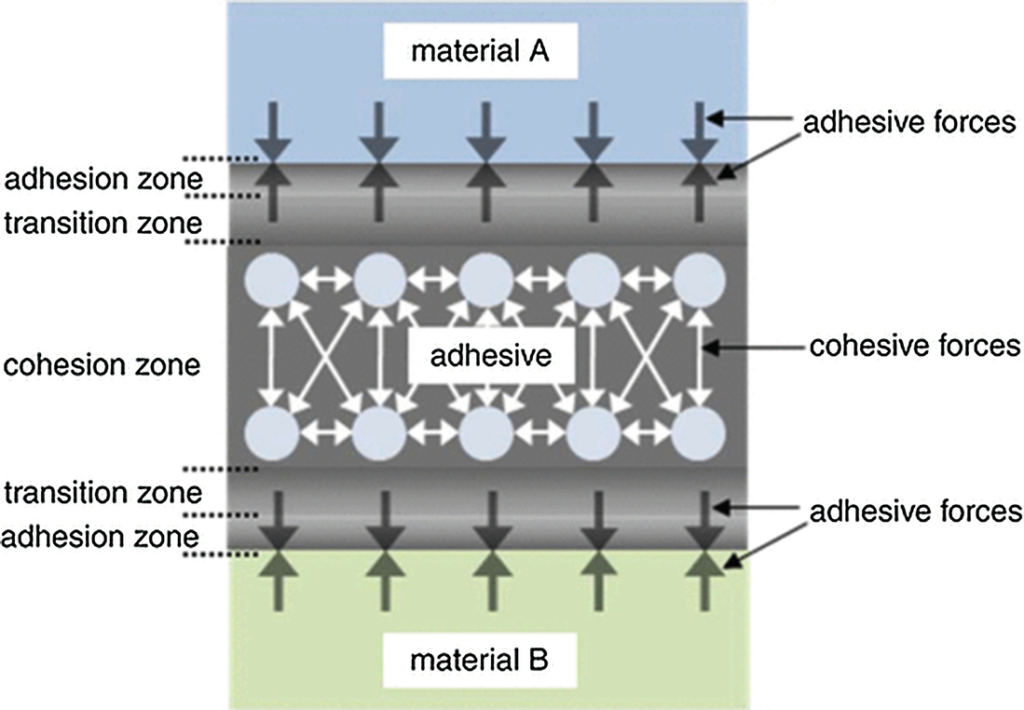
The Adhesive Zone
As mentioned above, the adhesive has a modified molecular structure in the adhesion zone due to bonding to the substrate surface. The phenomenon of adhesion is caused by molecular interactions between the substrate surface and the adhesive. A distinction can be made here between weak intermolecular interactions and strong chemical bonds.
Chemical bonds, however, only form for very few substrate/adhesive combinations, e.g. between silicone and glass, polyurethane and glass, and epoxy resin and aluminum. For some of these bonded joints it has been demonstrated that chemical bonds account for up to 50% of all the interactions. The long-term stability of these bonds depends directly on their resistance to moisture. In addition to the intermolecular and chemical adhesion forces, the bonding mechanism occasionally referred to as “micro-mechanical adhesion” can play a role, depending on the morphology of the substrate surface. This term is so-called because of the belief that an adhesive can effectively “mechanically cling” to a roughened substrate surface. “Micro-mechanical adhesion” is in general only considered to be of secondary importance. However if there are regular undercuts in the substrate – maybe even introduced by design – which the adhesive flows around, then this can increase the strength of the bonded joint.
The Transition Zone
The transition zone in which chemical, mechanical and optical properties of the adhesive are altered varies in thickness, from a few nanometers up to the millimeter range. The thickness depends on the nature of the substrate surface, the adhesive and the curing conditions. Where there are thick transition zones or thin bonded joints, the behavior of the entire bonded joint may be determined by the properties of the transition zone because in this case there is no cohesion zone.
The Cohesion Zone
In the cohesion zone, the adhesive possesses its nominal properties, as indicated on the data sheets. These properties are determined by the following molecular forces:
– The chemical bonds within the adhesive polymers;
– The chemical bonds resulting from crosslinking of the polymer;
– The intermolecular interactions between the molecules in the adhesive;
– The mechanical adhesion between various molecules in the adhesive.
These four cohesive forces affect the properties of the non-cured adhesive and determine for example the viscosity of the adhesive. The curing of the adhesive chiefly involves solidification of the adhesive via bonds between the molecules in the adhesive. This involves new bonds being formed (e.g. crosslinking of short chained molecules to form long chained molecules) and existing bonds being strengthened.
Both adhesion (including the transition zone) and cohesion play their part in maximizing the strength of a bond. Just as with a chain, the weakest link in a bonded joint determines what loads the joint can be subjected to.

